Episode 20: End Tidal Carbon Dioxide
Manage episode 186322525 series 1406855
Guest contributor: Lauren Weekes
https://phemcast.files.wordpress.com/2017/09/et-06092017-16-24.mp3
What is ETCO2?
- % or partial pressure of carbon dioxide measured somewhere near the mouth at the end of a normal exhalation (hence end tidal, end of tidal volume breath)
- To get a measurement the following systems need to be functioning:
- Metabolically active tissue to produce CO2
- Circulation & cardiac output to carry that CO2 to the lungs in blood
- Transfer of CO2 between the blood and the air in the lung
- Gas in and out of the lung to excrete the CO2
- Brilliant monitor in anaesthesia in that in elective cases, we start off with healthy patients are looking for deviations from the norm- and a normal ETCO2 trace tells you that all those components are functioning.
- It is still extremely useful in prehospital care, but we just have to remember that an abnormal trace or value may be caused by problems with one or more of those systems i.e circulation, gas exchange, ventilation (rarely tissue metabolism)
- Much better than pulse oximetry, because of the difference in lag time between clinical change occurring and being able to see it on the monitor- less than 3 seconds for sidestream capnography, compared to up to 90s for pulse oximetry
How does ETCO2 relate to arterial CO2?
- What we’re REALLY interested in is arterial CO2 as this is the clinically significant value in a number of clinical scenarios; for example in the brain-injured patient, we want to keep arterial CO2 values normal as we know that this determines the state of cerebral vasoconstriction or dilation, and thus affects ICP. In non-brain injured patients, high arterial CO2 can lead to a respiratory acidosis, and low pH values are harmful to most body tissues, in particular the clotting cascade (because of its reliance on enzymes, which function best in a narrow range of pH), and cardiac contractility.
- In healthy people, ETCO2 is usually 0.5-1kPA LOWER than the arterial value. Why is this? CO2 is only found in parts of the lung which participate in gas exchange, i.e are perfused with blood. So the trachea and first few generations of bronchi do not participate in gas exchange and are known as the dead space. They ARE however filling with gas during breaths, and as such gas from this dead space DILUTES the gas containing CO2 that has come predominantly from the alveoli.
- What we are assuming when we ask ETCO2 to substitute for arterial CO2 is that there is normal matching of ventilation to perfusion occurring in the lungs, so that all the mixed venous CO2 returning to the lungs from respiring tissue can equilibrate with alveolar gas and be eliminated via ventilation
What causes a discrepancy between arterial and ETCO2?
- Artefact
Loose connections, not having nasal prongs up nose, dilution with high oxygen flows (partic when using nasal prongs)
- Failure of venous CO2 to cross to ventilated alveoli
Alveolar dead space- alveoli are ventilated but not perfused
Classically low cardiac output states, PE, etc
- Failure of alveolar gas to be transported out of the lungs because alveoli are perfused but not ventilated (shunt):
- pneumonia and pulmonary edema, pulm haemorrhage (alveoli filled with fluid)
- tissue trauma: alveolar wall swelling
- atelectasis: collapse of alveoli from failure to expand, or absorption of the air out of the alveoli without replacing it
- mucous/vomit plugging
- Global ventilation failure e.g airway obstruction, hypoventilation esp where tidal volume is very low- dead space is fixed, so as a proportion of each breath it gets higher as tidal volume reduces until there is minimal ALVEOLAR ventilation
How do we measure it?
- Usually by infra-red absorption- CO2 absorbs infra-red light in a manner proportional to its concentration in the sampled gas.
- Can be measured from a breathing circuit attached to an invasive airway device e.g supraglottic airway or endotracheal tube, or from a number of methods in the spontaneously breathing patient, such as a specific nasal cannula, or a sampling tube attached to an oxygen mask. Important to note that the waveform, and values for ETCO2 are very different in the spontaneously breathing patient, and we’ll come back to that later.
- Might be measured directly from the breathing circuit (mainstream) or sucked out of the circuit in a sampling tube (sidestream).
- Might display results as a waveform with a value given for ETCO2, or simply a number (capnometry) although the latter much less useful.
- Colorimetric devices are available which change colour, based loosely on percentage of gas present. pH related. Occasionally used as an adjunct to waveform
What does the waveform mean?
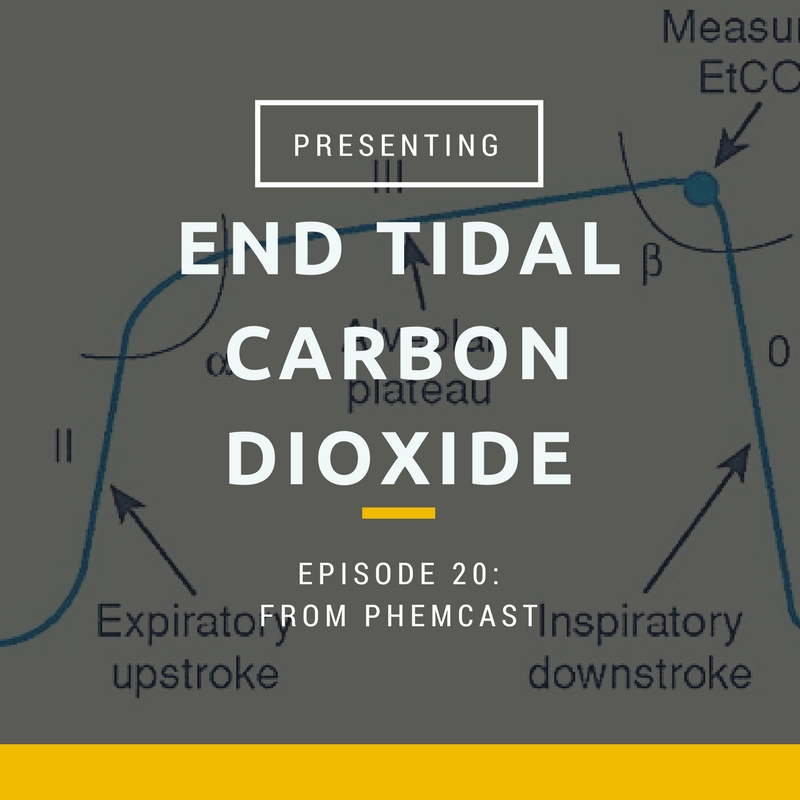
- The classic waveform that you will see in textbooks come from CO2 measured in the ventilated patient.
- The graph has time in seconds along the x axis and partial pressure in kPa along the y axis
- Phase I (inspiratory baseline) reflects inspired gas, which is normally devoid of carbon dioxide.
- Phase II (expiratory upstroke) is the transition between dead space and alveolar gas from the respiratory bronchioles and alveoli.
- Phase III is the alveolar plateau, when largely homogenous gas from the alveoli empties. This is the most accurate reflection of arterial co2
- Phase 0 is the inspiratory downstroke, the beginning of the next inspiration
- In the spontaneously breathing patient, there is not usually a plateau phase, which makes interpretation of ETCO2 values more difficult
EVIDENCE
ETT placement
- All major anaesthetic organisations mandate the use of ETCO2 to confirm ETT placement
- Good evidence that the trace is not completely flat even in cardiac arrest- Silvestri Ann Emerg Med 2005
- Should seen >7 waveforms to exclude oesophageal (Orinato 1993)
Cardiac arrest- general
- 2010 & 2015 ERC guidelines recommend use of waveform capnography
- Not new- 1978 paper Kalenda in Resuscitation described the use of capnogram as a guide to CPR efficacy
Predicting ROSC
- Grmec 2003 & 2011 in Critical Care
- ETCO2 of >2.4kpa after 20min predictive of rosc , <1.3 = no ROSC
- Alwens 2001 used cut off 10mmHg
- Systematic review in 2013 Resuscitation used cut off of 1.3kPa but this wasn’t 100% sensitive across all studies
- Concerns also raised by Norwegian paper in Resuscitation again 2011 showing a number of confounding factors made interpretation of etco2 problematic inc rhythm, bystander CPR, cause of arrest
CPR quality
- As noted in 1978, ETCO2 drops off when chest compressions become ineffective.
- Qvigstad et al showed in again in Resuscitation in 2013, confirming inter-individual variation in effectiveness of CPR using ETCO2 as a surrogate for CO
Trauma
- Deakin et al. (J. trauma 2004) showed that end-tidal CO2 may be of value in predicting outcome from major trauma (19). In a study of 191 blunt trauma patients, only 5% of patients with an end-tidal CO2 determination of 3.25 kPa survived to discharge
PRACTICAL USE
When should we use ETCO2 monitoring in the prehospital setting?
- Mandatory if intubating (RSI, cardiac arrest)
- Mandatory if performing procedural sedation where consciousness impaired
- Highly recommended in cardiac arrest
- Highly recommended in all critically ill patients
In cardiac arrest:
Attach to circuit/ BVM at soonest available opportunity
Use it to confirm intubation (if using)
Use it as a guide:
- If ETCO2 has been steady during CPR but then begins to fall, consider changing rescuer
- As corroborating evidence around decision making- if there has been no ROSC after 20min of full ALS protocol and ETCO2 remains below 1.3kPA, you are highly unlikely to resuscitate that patient
- If there is a sudden increase in ETCO2- well done, you’ve achieved ROSC (even if you can’t yet feel a pulse- in fact, maybe you needn’t do a pulse check if you’ve got ETCO2)
- Optimise ventilation post ROSC as you are now dealing with a head-injured patient.
In the critically ill patient:
- If I can only have one monitor on an entrapped patient, I’d pick capnography
- You will learn more quickly than any other method when your patient is deteriorating- e.g in blood loss, ETC02 will gradually fall. In the head injured patient who’s coning, you’ll see apnoeas and gradually rising ETCO2. In the heart failure patient who’s about to arrest, you’ll see their ETCO2 fall precipitously almost before anything else. In the comatose patient, you’ll be able to see that their airway is obstructed on the capnography a full 30 to 60s before their sats drop (by which point you’re already a long way down the oxygen dissociation curve).
- You can also see when your treatment is working- if you give a patient in septic shock some fluid and improve their CO, you’ll see a rise in ETC02
- You can confirm adequacy of respiratory function in the fitting or post-ictal patient when all other methods fail
PITFALLS
Device failure- lines blocking, batteries running out, pump failure.
Test by blowing
Over-interpreting the accuracy of non-invasive capnography
- Those lovely graphs showing curare clefts, rebreathing, bronchospasm etc you see on lots of different websites and in textbooks? They are almost all referring to capnography in the intubated and ventilated patient, who has a constant tidal volume.
- Numbers are often wildly inaccurate in the critically unwell population, and there may be an ET-arterial gradient of 10kPA.
- What CAN you tell from it? 1. Ventilation is occurring (accurate RR) 2. There is a cardiac output 3. You can interpret trends ie a gradual rise or fall in CO2, in the given clinical context 4. Very low is bad whichever way you look at it
Sometimes a low ETCO2 value is due to hyperventilation (because as we all remember, arterial CO2 concentration is almost linearly related to alveolar minute ventilation) BUT it may be hypoventilation with increased proportion of dead space ventilation compared to alveolar ventilation
Not using capnography
- The more you use it, the more familiar with various patterns you will become
- Stick it on everyone –it causes no harm. See what happens when you give a decent dose of morphine:
- slows respiratory rate but breaths are normal volume
- You get reduced alveolar MINUTE ventilation but normal alveolar TIDAL ventilation
- Therefore what you see at ETCO2 is reasonably representative of arterial concentration because the same number of alveoli are ventilated and have opportunity to equilibrate with the blood CO2
- This is unlike when a patient is making low tidal volume breaths, because then you’re largely ventilating dead space, and a much smaller number of alveoli are ventilated and thus equilibrium cannot occur between blood and gas
Demonstration traces:
From: Capnography Outside the Operating Rooms, Anesthes. 2013;118(1):192-201. doi:10.1097/ALN.0b013e318278c8b6
A Prolonged phase II, increased α angle, and steeper phase III suggest bronchospasm or airway obstruction.
B Expiratory valve malfunction resulting in elevation of the baseline, and the angle between the alveolar plateau and the downstroke of inspiration is increased from 90°. This is due to rebreathing of expiratory gases from the expiratory limb during inspiration.
C Inspiratory valve malfunction resulting in rebreathing of expired gases from inspiratory limb during inspiration (reference 5 for details).
D Capnogram with normal phase II but with increased slope of phase III. This capnogram is observed in pregnant subjects under general anesthesia (normal physiologic variant and details in reference 9).
E Curare cleft: Patient is attempting to breathe during partial muscle paralysis. Surgical movements on the chest and abdomen can also result in the curare cleft.
F Baseline is elevated as a result of carbon dioxide rebreathing.
G Esophageal intubation resulting in the gastric washout of residual carbon dioxide and subsequent carbon dioxide will be zero.
H Spontaneously breathing carbon dioxide waveforms where phase III is not well delineated.
I Dual capnogram in one lung transplantation patient. The first peak in phase III is from the transplanted normal lung, whereas the second peak is from the native disease lung. A variation of dual capnogram (steeple sign capnogram – dotted line) is seen if there is a leak around the sidestream sensor port at the monitor. This is because of the dilution of expired PCO2with atmospheric air.
J Malignant hyperpyrexia where carbon dioxide is raising gradually with zero baseline suggesting increased carbon dioxide production with carbon dioxide absorption by the soda lime.
K Classic ripple effect during the expiratory pause showing cardiogenic oscillations. These occur as a result of to-and-for movement of expired gases at the sensor due to motion of the heartbeat during expiratory pause when respiratory frequency of mechanical ventilation is low. Ripple effect like wave forms also occur when forward flow of fresh gases from a source during expiratory pause intermingles with expiratory gases at the sensor.
L Sudden raise of baseline and the end-tidal PCO2(PETCO2) due to contamination of the sensor with secretions or water vapor. Gradual rise of baseline and PETCO2occurs when soda lime is exhausted.
M Intermittent mechanical ventilation (IMV) breaths in the midst of spontaneously breathing patient. A comparison of the height of spontaneous breaths compared to the mechanical breaths is useful to assess spontaneous ventilation during weaning process.
N Cardiopulmonary resuscitation: capnogram showing positive waveforms during each compression suggesting effective cardiac compression generating pulmonary blood.
O Capnogram showing rebreathing during inspiration. This is normal in rebreathing circuits such as Mapleson D or Bain circuit.
Useful links:
https://lifeinthefastlane.com/ccc/capnography-waveform-interpretation/
http://www.capnography.com/new/index.php?option=com_content&view=article&id=131&Itemid=993
45 episodes




